Methodist Healthcare Adopts IR-MED’s PressureSafe™ Device for Usability Study: Decision Support Device Aims to Reduce Substantial Healthcare Burden of Pressure Injuries
- Patient enrollment and monitoring with PressureSafe™ has commenced
- In a similar study conducted at the world’s 2nd largest HMO, PressureSafe™ had efficacy of 92% in detection and the incidence of pressure injuries were reduced by 50% during the study
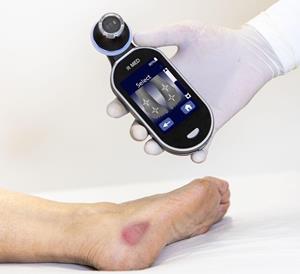
- A skin-color agnostic decision support device, PressureSafe™, uses an AI-based algorithm and infrared light to sense beneath the skin’s surface to detect biomarkers of pressure injuries
- $26.8 billion is spent each year on the prevention and treatment of pressure injuries, and pressure injuries directly result in the death of 60,000 people annually in the U.S.
Rosh Pina, Israel, Sept. 10, 2024 (GLOBE NEWSWIRE) -- IR-MED Inc., (“IR-MED” or the “Company”) (OTCQB:IRME), developer of a noninvasive artificial intelligence (AI) driven spectrographic analysis technology platform to address significant healthcare needs, announced today the start of a usability study for its lead product, PressureSafe™, at San Antonio, Texas based Methodist Healthcare. The study, titled “Safety and Efficacy of the PressureSafe Device for Early Detection of Pressure Injury in People with Various Skin Tones, Including Dark Skin Tones,” has received approval from Methodist Healthcare and has commenced patient enrollment and monitoring. Methodist Healthcare is widely regarded as one of the most respected healthcare providers in its region. With a growing network of care locations including hospitals, surgery centers, ERs, and family health clinics, each year Methodist Healthcare serves 608,000 patients, including 11,000 births, and 330,000 ER visits.
PressureSafe™, an innovative non-invasive medical device that uses infrared optical spectroscopy and an AI-based algorithm, is designed to effectively detect early-stage pressure injuries for all skin tones. PressureSafe™ is skin-color agnostic because it uses infrared light to detect biomarker changes below the skin’s surface. The decision support device is FDA listed.
Principal Investigator of the study and Director of the Professional Nursing Practice at Methodist Healthcare, Mary Lee Potter, PhD, MBA, RN, CWOCN, commented, “This is a very important study for our nurses and entire medical team, as we are continuously seeking to improve outcomes for our patients. We look forward to utilizing PressureSafe™ at Methodist Hospital Metropolitan and are eager to evaluate its potential to increase the accuracy of early pressure injury detection and prevention. As the device evaluates the tissues beneath the skin and digitally assesses biomarkers, the technology can objectively augment human visual inspection, and this is very interesting for us.”
The study aims to improve the early detection and prevention of pressure injuries among all patients. Importantly, the study aims to address the substantial challenge of healthcare inequality in the detection of pressure injuries in people of dark skin tones who are more than twice as likely to suffer from pressure injuries than those with lighter skin tone, according to a 5-year study published in Wounds. The current standard of care relies on visual inspection of the skin, which can be less effective for early detection in individuals with darker skin tones.
Up to 104 people will be enrolled in the study, approximately half with dark skin tones. Registered nurses specialized in wound care (WOCN) will be trained in using PressureSafe™. Sensitivity and specificity will be assessed and compared to standard of care visual skin assessment done by the WOCN nurses.
“As our first major usability study in the United States, this marks a major milestone for IR-MED and PressureSafe™. We couldn’t be more pleased to partner with the Methodist Healthcare and Principal Investigator Dr. Potter,” stated Dr. Yaniv Cohen, IR-MED’s Co-Founder and Chief Science Officer. “At this highly prestigious institution, we hope to demonstrate that PressureSafe™ can provide support for nursing staff, significantly improve patient outcomes, and reduce healthcare costs.”
IR-MED’s prior usability study for PressureSafe™ was conducted at two hospitals in Israel owned by Clalit, Israel’s largest health maintenance organization (HMO) and the second largest in the world. PressureSafe™ detected pressure injuries with 92% sensitivity and 88% specificity, while the incidence of pressure injuries was reduced by 50% during the study period.
According to the National Pressure Injury Advisory Panel, in the U.S. alone, 60,000 patients die every year as a direct result of pressure injuries. Patient care cost per pressure injury ranges from $20,900 up to $151,700, for the 2.5 million patients per year who develop pressure injuries. Pressure injuries are one of the five most common harms experienced by patients and the second most common claim for lawsuits after wrongful death.
About IR-MED
IR-MED Inc., is developing a noninvasive spectrographic analysis technology platform, allowing healthcare professions to detect, measure and monitor, in real time, different molecules in the blood, in human tissue, and in body fluids without invasive procedures. PressureSafe™, the first product planned to be launched, is a handheld optical monitoring device that is being developed to support early detection of pressure injuries (PI) to the skin and underlying tissue, regardless of skin tone as it calibrates personally to each patient’s skin.
IR-MED’s technology is being developed to allow accurate readings of biomarkers in a non-invasive method, that may provide caregivers the optimal decision support-system in cases where uncertainties disturb physicians in their decision processes.
IR-MED holds patents protecting its technology and innovations in the noninvasive tissue analysis, and in the modeling and analysis of subcutaneous tissue.
PressureSafe™ is currently undergoing usability studies at multiple medical centers.
Safe Harbor Statement / Forward-Looking Statements
Statements included in this press release, which are not historical in nature, are forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. For example, IR-Med is using forward looking statements when it states that its technology platform, and specifically the PressureSafe device, are increasingly being recognized by medical practitioners and technologists for its potential to make a significant contribution in healthcare by reducing the risk and severity of pressure injuries. Statements relating to the future performance of IR-Med are subject to many factors including, but not limited to, the sufficiency or working capital and our ability to raise the capital needed to fund our development efforts, completion of the development and design of PressureSafe device, results of clinical/useability studies and trials, timing of product development, FDA approval/clearance of products in development, customer acceptance of our products in the market, the introduction of competitive products, the impact of any product liability or other adverse litigation, commercialization and technological difficulties, and the other risks identified in our most recent annual report on Form 10-K filed on March 29, 2023 with the Securities and Exchange Commission. Such statements are based upon the current beliefs and expectations of management and are subject to significant risks and uncertainties. Actual results may differ from those set forth in the forward-looking statements. The forward-looking statements contained in this press release are made as of the date hereof, and we do not undertake any obligation to update any forward-looking statements, whether as a result of future events, new information, or otherwise.
Contact:
Sharon Levkoviz, Chief Financial Officer
Tel: +972 (0) 4 6555054
Attachment
Source: IR-Med, Inc.
Released September 10, 2024